Chapter 2
MATTER & ENERGY
Man's first application of the laws of governing matter and energy was his invention of
the basic machines: The Disinclined Plane, The Blocked Tackle, The Leveraged Buyout,
The Wheelie and The Screw-Up. This was a great step forward for civilization, but
it involved only an intuitive understanding of mechanics. An explanation of the
underlying principles came with the rise of experimental science.
Galileo and Gravity
Galileo's demonstration of the laws of gravity is a perfect example of the use of the scientific method. For a thousand years before Galileo, people had accepted the theory of gravity propounded by Aristotle.Aristotle alleged that heavy and light objects fell at the same rate. To explain the obvious exceptions, he invoked an ideal state he called a "vacuum." Only in this imaginary and purely theoretical state could the "true" behavior of falling objects be observed.
Galileo overturned this idealized theory in a famous experiment. He dropped a feather and a lead weight from the top of the Leaning Tower of Pisa. The feather drifted down slowly while the weight plummeted quickly. Thus, by the use of the experimental method, Galileo showed that heavy objects fall faster than light ones.
Newton's Laws
Isaac Newton also used direct observation to formulate his laws.Newton was in government service for many years. His first law states:
- A body at rest tends to remain at rest, while a body in motion at a constant velocity in a straight line tends to continue in that motion.
Clearly, this law is based on first-hand observation of a bureaucracy in action.
Once night, Newton became engaged in a heated argument at a local bar over a question
of epicycles, leading him to punch his opponent in the nose. After being thoroughly
worked over, Newton comtemplated the results and announced his next law:
- Every action has an equal and opposite reaction.
In a well-known story, Newton discovered gravity when he was hit on the head while sitting under an apple tree. This tale is, of course, fictitious. It was actually a fig tree, and the result was his best-known theory:
- I bet you could make a swell cookie out of these figs.
The Elements
|
The early alchemists thought that there were only four kinds of matter, or elements
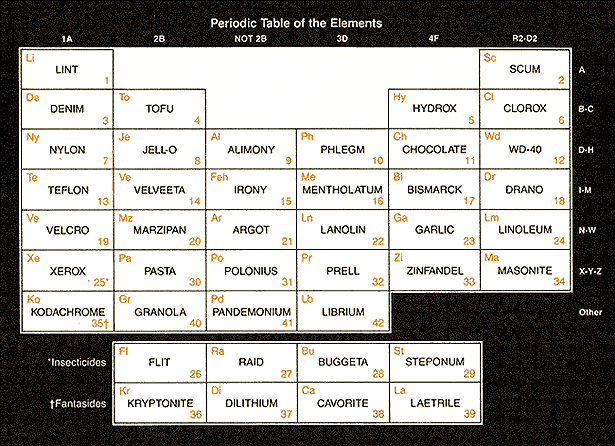
- Earth, Air, Fire and Water. All of the different kinds of matter we see around us were believed to come from mixtures of these four. While this was a good start, four elements alone did not seem to porvide enough diversity to account for all matter. Today we recognize far more elements than the ancients, and can arrange them in a periodic table (below) to make them appear impressive and hard to understand. | 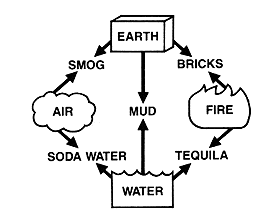 |
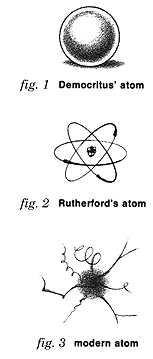
The AtomThe elements are made up of still more basic units called atoms.The earliest theories held that the atom was hard, round and indivisible, like a school lunch meatball. (fig. 1)
In the earlier 20th century, it was discovered that atoms consisted of three smaller
particles. This was tolerable, and it made for a nice graphic symbol for corporate logos.
(fig. 2) Today, things have gone seriously downhill. Our modern picture of the atom has lines zinging off in all directions (fig. 3) to represent the literally hundreds of subatomic particles that have been discovered. The atom is represented as blur on account of Heisenberg's Uncertainty Principle, which says you can't really know where anything is.* *A full discussion of Heisenberg's Uncertainty Principle may be found in the Appendix. Then again, it may not. |
 |
|
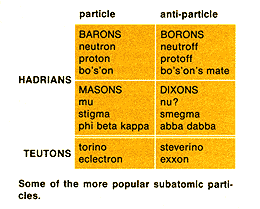
The proliferation of particles (at right) has led to an attempt to simplify the
system. Thus it is now theorized that all subatomic particles are actually a single
particle, called a quack, with widely variable properties, including its
anti-particle, the quirk. Shown below are the currently understood properties of the quack. On a side note: an amusing though less plausible parody of this material can be found in any book on current particle physics. |
 |
The First Nuclear Reactor
The Manhattan Project, which resulted in the first sustained nuclear reaction, was one of two top-secret World War II experiments. Through a clerical error, it was inadvertently located in Chicago.Its sister program, the Chicago Project, headquartered in Manhattan, was originally thought the more promising. The goal was a machine that would convert electrical energy into Uranium 235. Dropped on German cities, the device would cripple the Nazi war machine by absorbing all the electrical power from nearby manufacturing plants and communications centers, and further create confusion by blocking roads with the heaps of Uranium it produced.
To everyone's surprise, it was the Manhattan group that succeeded, creating a device that could generate electrical power from the breakdown of Uranium. The competing Chicago Project is now only a footnote to history.
Relativity
The principles of Eisenstein's relativity are too well known today to require explanation. The humblest reader of this book can undoubtedly give a clear and concise account of such relativistic phenomena as the Michealson-Morley experiment, the Lorentz-Fitzgerald contraction and the Dow-Jones Average. The familiar World-Line Diagram (below) sums it all up.
The Universe / Matter & Energy / The Earth / Evolution / The Descent of Man